
Endometriosis Hereditary Risk Calculator
This tool estimates your relative risk of developing endometriosis based on your family history. It uses data from large-scale genetic studies to give you a personalized risk assessment.
Family History
Additional Factors
When you hear the word "hereditary" you might picture a trait passed down like eye colour or a talent for playing piano. But does that idea apply to a painful condition like Endometriosis is a chronic gynecological disorder where tissue similar to the uterine lining grows outside the uterus, causing pelvic pain, heavy periods and infertility? Researchers have been digging into the DNA code for years, trying to decode whether the disease runs in families or if it’s mostly driven by other factors. The short answer: genetics plays a definite role, but it’s only part of a bigger puzzle.
TL;DR
- Endometriosis affects about 1 in 10 women of reproductive age.
- First‑degree relatives have roughly a 2‑3× higher risk.
- Specific genetics endometriosis markers (e.g., WNT4 gene, a regulator of uterine development) raise susceptibility.
- Most risk comes from a mix of many small‑effect genes, epigenetic tweaks and environmental influences.
- If you have a family history, consider genetic counseling and early symptom monitoring.
What Exactly Is Endometriosis?
Endometriosis occurs when endometrial‑like tissue implants on the ovaries, fallopian tubes, bowel or even the diaphragm. Each month that tissue bleeds, it has nowhere to exit the body, leading to inflammation, scar tissue and chronic pain. The condition is diagnosed by laparoscopy, imaging or, in some cases, clinical symptom patterns. Key symptoms include:
- Sharp or throbbing pelvic pain, especially during periods.
- Infertility or difficulty conceiving.
- Heavy or irregular menstrual bleeding.
- Pain during sex or bowel movements.
Because the symptoms overlap with other conditions (like pelvic inflammatory disease or ovarian cysts), many women experience years of misdiagnosis.
How Genetics Influences the Disease
Broadly, Genetics refers to the study of DNA sequences that determine inherited traits. In endometriosis, the genetic contribution is polygenic, meaning dozens to hundreds of tiny variations across the genome collectively push the odds higher.
Two concepts are crucial:
- Single‑nucleotide polymorphisms (SNPs): tiny changes in a single DNA base that can affect how a gene works. Thousands of SNPs have been linked to endometriosis risk in large genome‑wide association studies (GWAS).
- Epigenetic modifications: chemical tags that turn genes on or off without altering the DNA code itself. Factors like chronic inflammation or hormonal exposure can reshape these tags, influencing disease severity.
Key Genetic Findings From Recent Research
Since the first GWAS on endometriosis in 2012, scientists have identified more than 20 risk loci. Below is a snapshot of the most replicated genes and what they do:
| Gene / Locus | Biological Role | Odds Ratio (OR) | Study Sample (n) |
|---|---|---|---|
| WNT4 - a signaling molecule in uterine development | Regulates estrogen‑driven cell growth | 1.45 | 27,000 (European ancestry) |
| VEZT - involved in cell adhesion | May affect implantation of ectopic tissue | 1.32 | 22,500 (mixed ancestry) |
| GREB1 - estrogen‑responsive growth regulator | Amplifies estrogen signalling | 1.28 | 30,000 (multi‑ethnic) |
| FN1 - fibronectin, extracellular matrix protein | Promotes tissue adhesion and scar formation | 1.24 | 19,800 (European) |
Notice the odds ratios cluster around 1.2-1.5. That tells us each variant nudges risk only slightly; it’s the cumulative effect of many variants that matters.
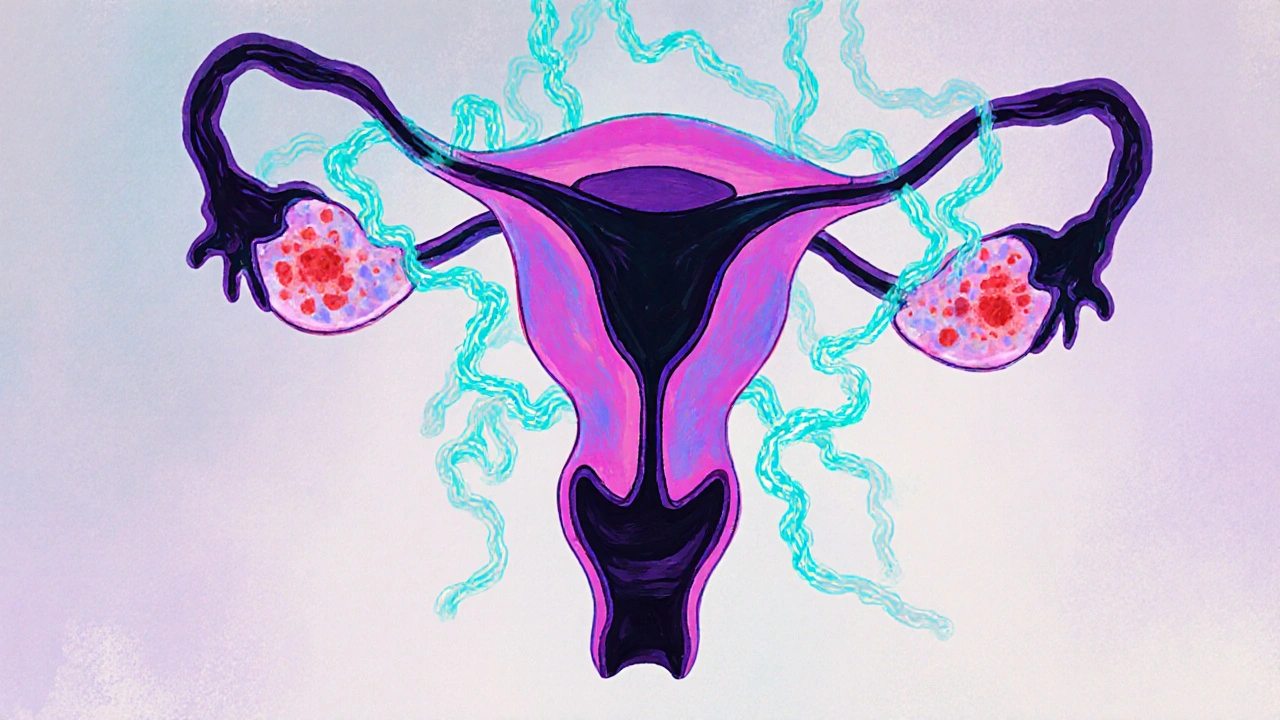
Is Endometriosis Hereditary? Family‐Based Evidence
When researchers talk about Hereditary risk, they usually mean that first‑degree relatives (mother, sister, daughter) share a higher likelihood of developing the condition compared to the general population.
Large twin and family studies provide the numbers:
- Sibling concordance rates hover around 20‑25% in population samples.
- Twin studies estimate heritability at roughly 50%, meaning half of the variation in disease risk is genetic.
- A 2021 meta‑analysis of 12 cohort studies found that women with an affected mother or sister had a 2.3‑fold increased risk.
These figures confirm a hereditary component but also highlight that many women with a strong family history never develop symptoms, underscoring the role of non‑genetic triggers.
Interpreting Your Own Family History
If you’re wondering whether your mom’s painful periods or your aunt’s infertility hints at a genetic link, consider these steps:
- Chart the family tree. Note any relatives (especially maternal side) diagnosed with endometriosis, chronic pelvic pain, or unexplained infertility.
- Assess age of onset. Early‑onset cases (before age 30) often signal a stronger genetic influence.
- Look for patterns. Multiple affected generations raise the suspicion of inherited risk.
With this information in hand, you can have a more informed conversation with your gynecologist or a genetic counselor.
Practical Steps: Testing, Counseling, and Lifestyle Choices
At present, there is no commercial DNA test that can definitively predict endometriosis. However, a few pathways exist:
- Research‑grade polygenic risk scores (PRS). Some academic labs offer PRS calculations for research participants. These scores combine many SNPs into a single risk number, but they’re not yet validated for clinical decision‑making.
- Genetic counseling. If you have multiple affected relatives, a counselor can discuss the heritability data, help you interpret any available genetic testing, and guide family planning.
- Early symptom monitoring. Women with a known family history should track menstrual pain, bleeding patterns and fertility concerns from their teens. Earlier detection often leads to less invasive treatment.
- Lifestyle adjustments. While genetics set the stage, factors like diet, stress, and exposure to endocrine‑disrupting chemicals can modulate gene expression (epigenetics). A Mediterranean‑style diet, regular exercise and minimizing plastics may help keep inflammatory pathways in check.
Emerging Therapies Targeting the Genetic Landscape
Scientists are turning genetic insights into new treatments:
- Selective progesterone receptor modulators (SPRMs). By tweaking hormone signalling pathways that interact with risk genes like GREB1, these drugs aim to shrink ectopic tissue without full‑blown hormonal suppression.
- RNA‑based therapies. Early‑phase trials are investigating small interfering RNAs (siRNAs) that silence over‑expressed genes such as FN1, potentially reducing scar formation.
- Epigenetic drugs. Molecules that reverse harmful DNA methylation patterns are being tested in animal models, hoping to restore normal gene expression in the pelvic environment.
While still experimental, these approaches illustrate how each genetic clue could eventually become a therapeutic target.
Frequently Asked Questions
Can a DNA test tell me if I will get endometriosis?
Not yet. Current commercial tests don’t have enough predictive power. Research‑grade polygenic risk scores exist but are not approved for clinical use.
If my mother has endometriosis, am I guaranteed to develop it?
No. Having an affected first‑degree relative raises your risk about two‑to‑three times, but many women with a family history never experience symptoms.
Should I see a genetic counselor?
If you have multiple relatives with endometriosis or early‑onset disease, a counselor can help you understand risk, discuss testing options and plan for family building.
Do lifestyle changes affect my genetic risk?
Yes, indirectly. Diet, exercise and reducing exposure to endocrine‑disruptors can influence epigenetic marks, which may lessen symptom severity even if the underlying genetic risk remains.
Are there any new medications that target genetic pathways?
Clinical trials are testing SPRMs, RNA‑based therapies and epigenetic drugs that specifically interact with genes linked to endometriosis. Results are promising but not yet widely available.





Holly Hayes
September 30, 2025 AT 13:03Honestly, the whole genetics thing sounds like a boutique buzzword for the biotech crowd. It’s like they took a fancy term and slapped it on a messy condition. The studies do show some family link, but it’s far from a crystal‑clear inheritance pattern.
Matthew Shapiro
October 4, 2025 AT 11:30Actually, the data suggests a modest increase in risk for first‑degree relatives, roughly two to three times higher. It’s not deterministic, just a statistical tilt. Keeping an eye on symptoms early can make a big difference in management.
Julia Phillips
October 8, 2025 AT 09:57I remember my cousin being dismissed for years before someone finally diagnosed her with endometriosis. The pain was relentless, and it took a lifetime of doctors to connect the dots. Knowing there’s a hereditary component gave us a reason to push for thorough testing sooner rather than later.
Richa Punyani
October 12, 2025 AT 08:23Dear readers, it is essential to consider both genetic predisposition and environmental triggers when evaluating risk. A thorough family pedigree, especially noting early‑onset cases, can guide clinical discussions. Additionally, lifestyle modifications such as reducing exposure to endocrine disruptors may mitigate epigenetic activation of risk genes.
Bhupendra Darji
October 16, 2025 AT 06:50Building on that, I would suggest documenting any maternal or sister history alongside menstrual patterns. This dual approach helps genetic counselors provide a clearer picture and may influence early intervention strategies.
Robert Keter
October 20, 2025 AT 05:17When we dive into the polygenic architecture of endometriosis, we quickly realize it is not a single‑gene disorder but a tapestry woven from dozens of modest effect loci. The WNT4 locus, for instance, nudges estrogen‑driven proliferation, while VEZT influences how ectopic cells adhere to pelvic surfaces. GREB1 amplifies hormonal signaling, and FN1 contributes to the extracellular matrix scaffolding that facilitates lesion stability.
These genetic pieces each contribute odds ratios in the 1.2‑1.5 range, meaning no individual variant can predict disease on its own. Instead, the cumulative polygenic risk score (PRS) aggregates small effects to stratify women into low, moderate, or high risk categories.
Family studies corroborate this, showing a heritability estimate of roughly 50%, akin to traits like height. Twin concordance rates hovering around 20‑25% underscore the genetic component, yet the fact that many women with affected relatives remain symptom‑free points to crucial environmental and epigenetic modifiers.
Epigenetics, the reversible chemical tags on DNA, can be altered by chronic inflammation, diet, and exposure to endocrine‑disrupting chemicals such as BPA. These modifications may up‑regulate pathogenic genes or silence protective pathways, effectively reshaping the risk landscape without changing the underlying DNA sequence.
From a clinical standpoint, the lack of a commercial diagnostic test reflects our current inability to translate these genomic insights into a reliable predictive tool. Research‑grade PRS can be calculated in academic settings, but they are not yet validated for routine care.
Nevertheless, knowing you have a family history should prompt proactive symptom monitoring-tracking menstrual pain intensity, bleeding patterns, and fertility concerns from adolescence onward.
Lifestyle interventions, including a Mediterranean‑style diet rich in omega‑3 fatty acids, regular aerobic exercise, and minimizing plastic use, may attenuate inflammatory pathways and potentially modulate epigenetic marks.
Therapeutically, the field is exciting: selective progesterone receptor modulators (SPRMs) aim to tweak hormone pathways implicated by genes like GREB1, while early‑phase RNA interference trials target overexpressed genes such as FN1 to reduce scar tissue formation.
Epigenetic drugs that reverse harmful methylation patterns are also on the horizon, offering a novel avenue to reset aberrant gene expression.
In summary, endometriosis is a multifactorial disease where genetics set the stage, but environment, epigenetics, and lifestyle dictate the final act. Understanding this interplay empowers women and clinicians to adopt a more personalized, preventive approach.
Rory Martin
October 24, 2025 AT 03:43One has to wonder why the pharma industry is so swift to push hormone suppressors while ignoring these genetic clues. It feels like a coordinated effort to keep us dependent on expensive pills rather than funding real gene‑targeted research.
Maddie Wagner
October 28, 2025 AT 01:10Let’s remember that knowledge is power. If you suspect a hereditary link, schedule a consultation with a genetic counselor- they can help you interpret family patterns and discuss any emerging testing options.
Boston Farm to School
October 31, 2025 AT 23:37Cool tip! 👍 Tracking symptoms on a simple spreadsheet can make the doctor’s visit much clearer.
Emily Collier
November 4, 2025 AT 22:03From a holistic perspective, integrating mental health support is vital. Chronic pelvic pain often leads to anxiety and depression, which in turn can amplify pain perception. A multidisciplinary approach that includes physiotherapy, nutrition counseling, and psychological care tends to yield better outcomes.
Catherine Zeigler
November 8, 2025 AT 20:30Adding to that, many patients find relief through low‑impact exercise like swimming or yoga, which can improve pelvic circulation without stressing joints. Also, supplementing with omega‑3 fatty acids and curcumin has shown some anti‑inflammatory benefits in small studies. It’s worth discussing these options with your provider to create a personalized regime.
henry leathem
November 12, 2025 AT 18:57The data unequivocally demonstrates that endometriosis is a prototypical polygenic disorder, where each locus contributes a minuscule effect size. Clinicians must therefore eschew reductionist diagnostics in favor of integrative risk modeling.
jeff lamore
November 16, 2025 AT 17:23Indeed, adopting an evidence‑based, patient‑centered framework aligns with best practice guidelines.
Kris cree9
November 20, 2025 AT 15:50this article is just a bunch of hype.
Paula Hines
November 24, 2025 AT 14:17When we examine the ontological underpinnings of disease causality, it becomes evident that the reductionist paradigm fails to capture the emergent properties of a system as complex as the human reproductive tract. By privileging genetic determinism, we obscure the multifactorial reality wherein socioeconomic status, environmental pollutants, and psychosocial stressors co‑act with polymorphic variants to shape phenotypic expression. Such a myopic view not only stalls therapeutic innovation but also perpetuates a narrative that blames individuals for their biological misfortune. A paradigm shift toward systems biology, integrating omics data with exposomic and behavioral datasets, is indispensable if we are to devise interventions that are both precise and equitable. In this light, the burgeoning field of epigenetic therapeutics offers a promising avenue to re‑program maladaptive gene expression patterns without altering the genome itself.
John Babko
November 28, 2025 AT 12:43Indeed, the synthesis of genetics, environment, and lifestyle, with a focus on holistic patient care, should be the cornerstone of future research and clinical practice.
Roger Perez
December 2, 2025 AT 11:10Hope everyone dealing with this feels less alone. 🌟 Remember: knowledge + community = strength!
michael santoso
December 6, 2025 AT 09:37While community support is valuable, let’s not forget that quantitative risk assessment models can provide an objective baseline, which is essential for allocating healthcare resources efficiently.
Jada Singleton
December 10, 2025 AT 08:03Honestly, many of these so‑called “studies” are just academic fluff that doesn’t translate to real‑world relief.
Emily Rossiter
December 14, 2025 AT 06:30Even if the science feels distant, staying informed and proactive can empower you to advocate for better care and support.